100 mL of 0.1 M CH3COOH, 50 mL of 0.1 M HCl is mixed with 50 mL of 0.1 M Ba( OH)2. The pH of mixture of solution is (pK, of CH3COOH is 4.74)
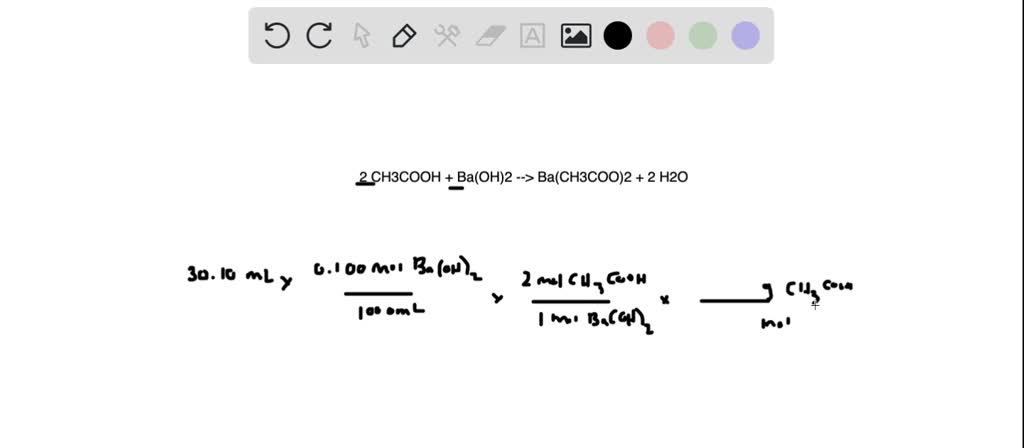
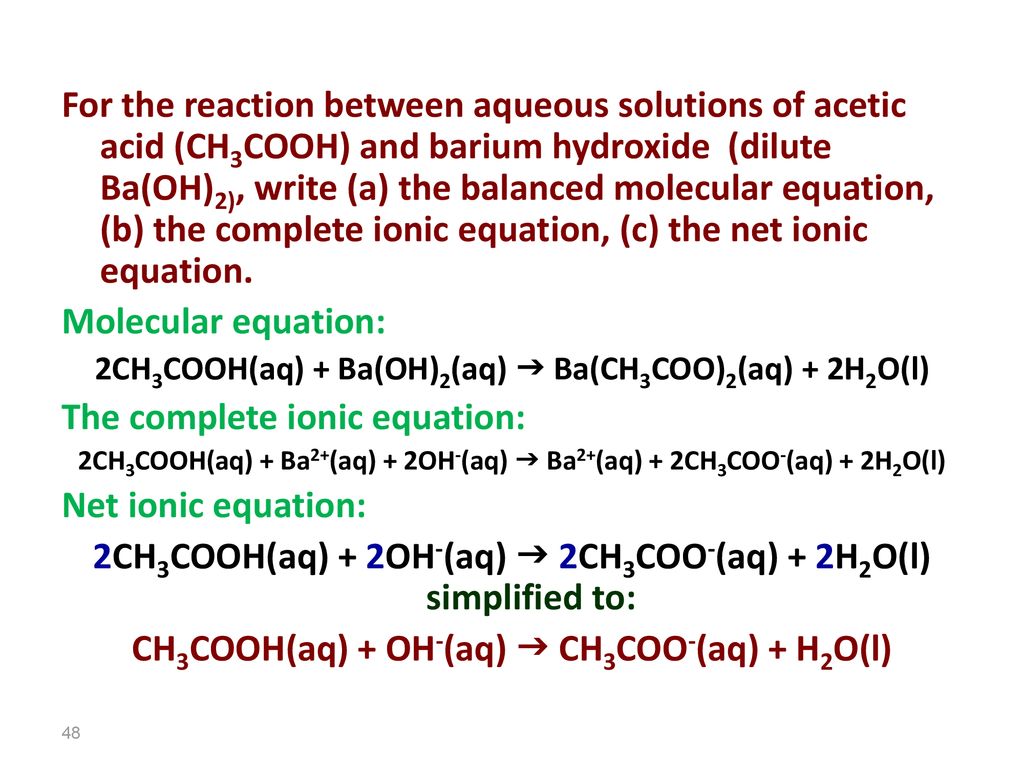
SOLVED: Enter the molecular equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced molecular equation. Identify all of the phases in your answer: CH3COOH(aq) +

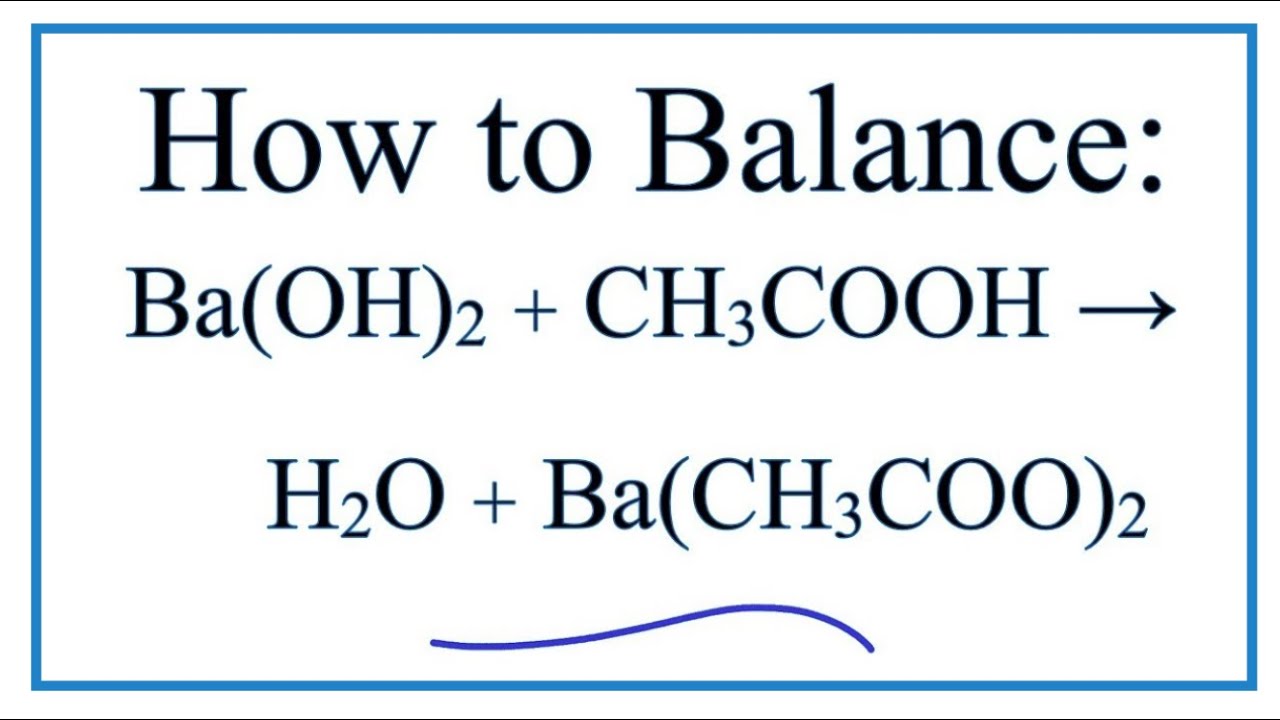
The complete balanced equation for the reaction between barium hydroxide and acetic acid is - brainly.com

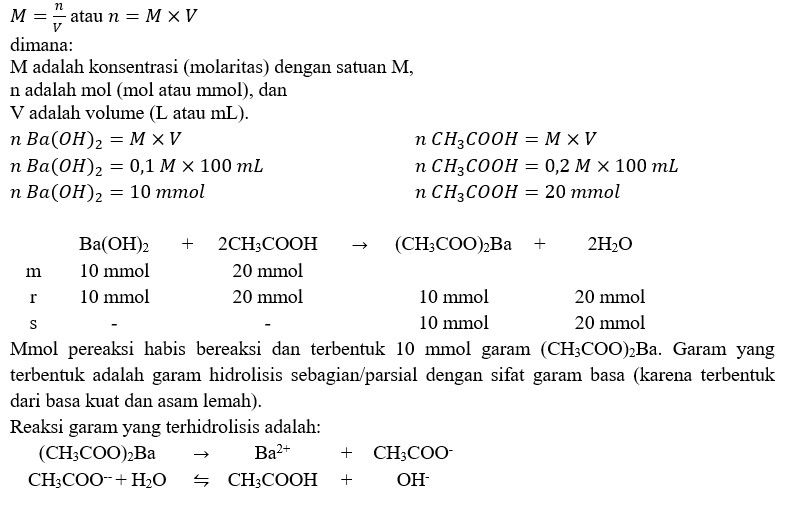
Sebanyak 400ml larutan CH3COOH 0,2M direaksikan dengan 200ml larutan Ba(OH)2 0,1 M. Jika Ka 2•10'-5 . - Brainly.co.id

SOLVED: Question 10 Not yet answered 100.0 mL of 0.10 M CH3COOH are mixed with 80.0 mL of 0.10 M Ba(OH)2. What determines the pH of the solution after the reaction? Marked

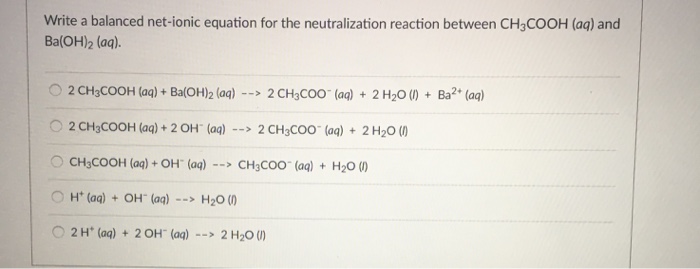
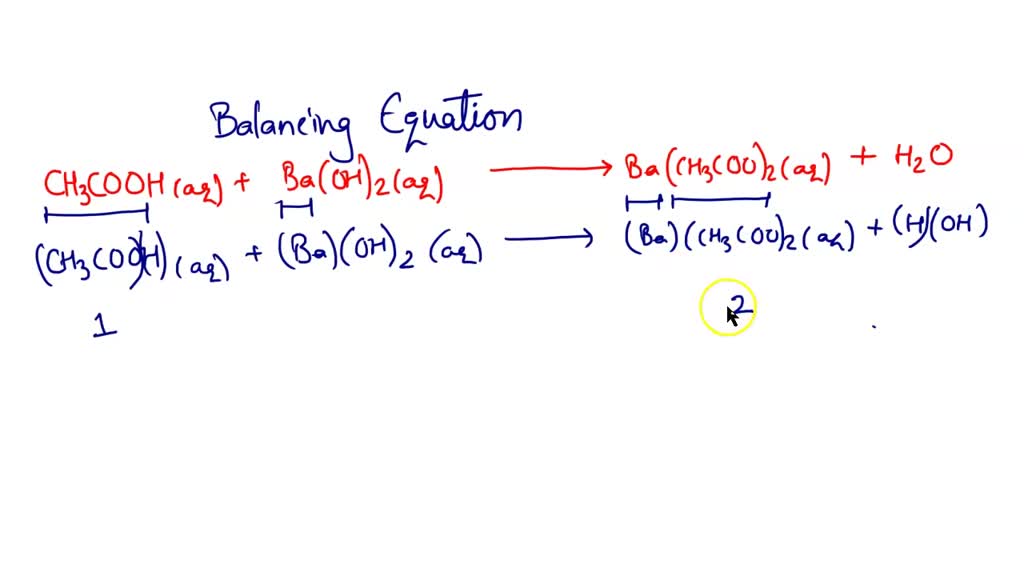
SOLVED: Acetic acid reacts with barium hydroxide. What is the sum of the coefficients for the reactants and products?

sebanyak 50 mL larutan Ba(OH)2 dapat dinetralkan dengan 20 mL larutan CH3COOH 0,1M. tentukan massa - Brainly.co.id

SOLVED: Enter the molecular equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced molecular equation. Identify all of the phases in your answer: CH3COOH(aq) +